We’ve just launched a new service.
Improve your Health AI System. Gain Access to the full text of: Clinical Trial docs, FDA’s Medical Devices, and Drug Approvals.
We can improve your organization’s health-focused AI systems by integrating the full text of over 220,000 individual PDFs with 4.7 Million unique pages of content.
From ClinicalTrials.gov and the US Food and Drug Administration.
Benefit from human-generated text not easily available elsewhere.
This is high-quality, rich, realistic content.
Carefully crafted for regulatory purposes.
Download image below for full details..
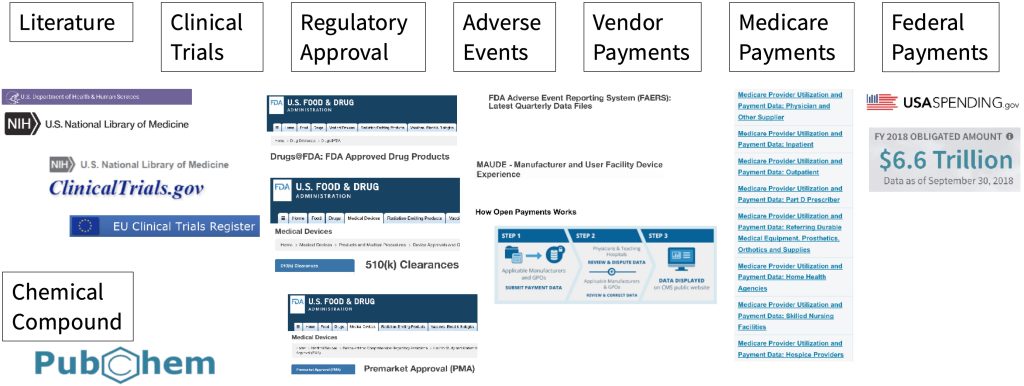
Everything you need to stand out in the crowded AI market
Quickly implement a significant AI market differentiator using better content sources.
Better Results
Most of the available content in the Internet has already been ingested by LLMs. We offer your organization relevant, accurate, human-written content. That’ll give your users better results.
Build vs. Buy
Your organization will avoid writing and maintaining data-acquisition and ETL code for Open Data. This is a done-for-you, hands-free, “data concierge” service. And it’s available now.
Collaboration
Tap our Open Data experts’ knowledge. This will help your team fully understand the richness of the Open Data ecosystem. Then we’ll design a solution to best fit your organization’s needs.
Fit for your Organization
Your team can quickly determine what data sources are a best fit for your use case. Our platform will then generate customized files, to match your backend, with the selected data.
Predictable Annual Flat Fee
Our pricing model is simple and transparent: your organization pays a flat annual fee, with unlimited internal use of the content. The fee includes quarterly updates and tech support.
Easy Integration
Working with your technical team, our data experts will ensure a quick, simple, and painless integration. Your system will ingest our content within 10 working days of signing the contract.
ClinicalTrials.gov
60,000 Clinical Trial docs
We wrote custom code to acquire and process Protocols, Statistical Analysis Plans (“SAPs”) and Informed Consent Forms (“ICFs”) from ClinicalTrial.gov
Our platform downloaded 60,000 individual PDFs with 2.7 Million unique pages.
On the right you see the first page of a full clinical trial Protocol.
The full Protocol is available here.
A sample Statistical Analysis Plan (“SAP) is available here.
Sample Informed Consent Forms (“ICFs”) are also available.


FDA – Medical Devices – 510(k)s
92,000 510(k)s
From the FDA’s website we acquired and processed approval documents for medical devices called “510(k)s”.
We have over 92,000 individual PDFs with 660,000 unique pages.
Please notice on the left the first page of an initial regulatory approval document. The full 510(k) is available here.
FDA – Medical Devices – PMAs
2,500 PMAs
The FDA has a second path to medical device approvals, through what are called “PMAs”. We acquired and processed those approval documents: over 2,500 individual PDFs with 75,000 unique pages.
On the right you see the first page of an initial regulatory approval document. The full PMA is available here.
We also have Instructions for Use (“IFUs”) / Manuals available. A sample IFU document is available here (warning: large file).


FDA – Drug Approvals
67,000 Drug Approvals
In terms of drugs, we acquire and process approval documents from the FDA’s CDER website and we currently hold over 67,800 individual PDFs representing 1.3 Million unique pages.
On the left you see the first page of an initial regulatory approval document. The full document is available here.
We also have supplemental filings. A sample is here.
Pricing
Simple, transparent pricing
Choose the right pricing plan that best fits your organization’s needs.
A one-time license fee for unlimited future use is also available. And/or for external use as well.
Package A: Text Only
$25,000
/year
Internal use only
- Extracted text from 200,000 PDFs
- Over 04.7 Million pages of text
- Available as database load files
- Integrated within a few weeks
- Personalized tech support
Package B: Text + PDFs
Most Popular
$50,000
/year
Internal use only
- Everything in Package A
- All individual PDFs file are provided
- User-defined customized file formats
- Personalized consulting sessions
- Unlimited tech support
Package C: Text + PDFs + other Open Data
Contact Us
External use allowed
- Everything in Package B
- Medicare Reimbursement data
- Medicare’s Open Payments
- NIH’s datasets: PubMed, UMLS
- European Medicines Agency’s data
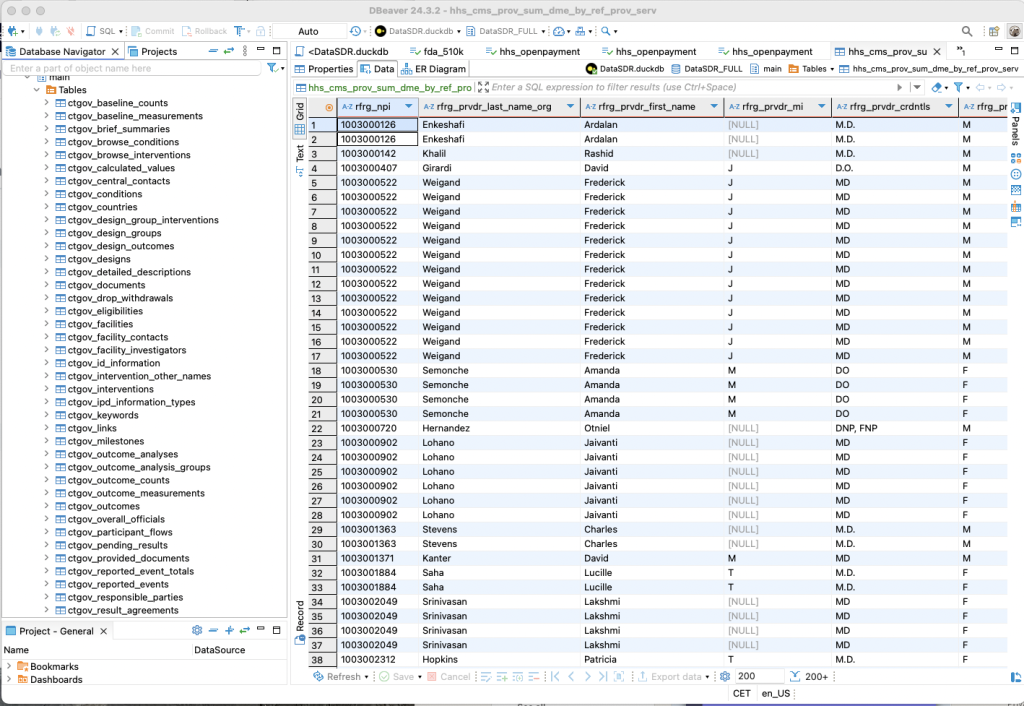
Below please find a screenshot of a database query tool showing some of the raw datasources already stored in our repository.
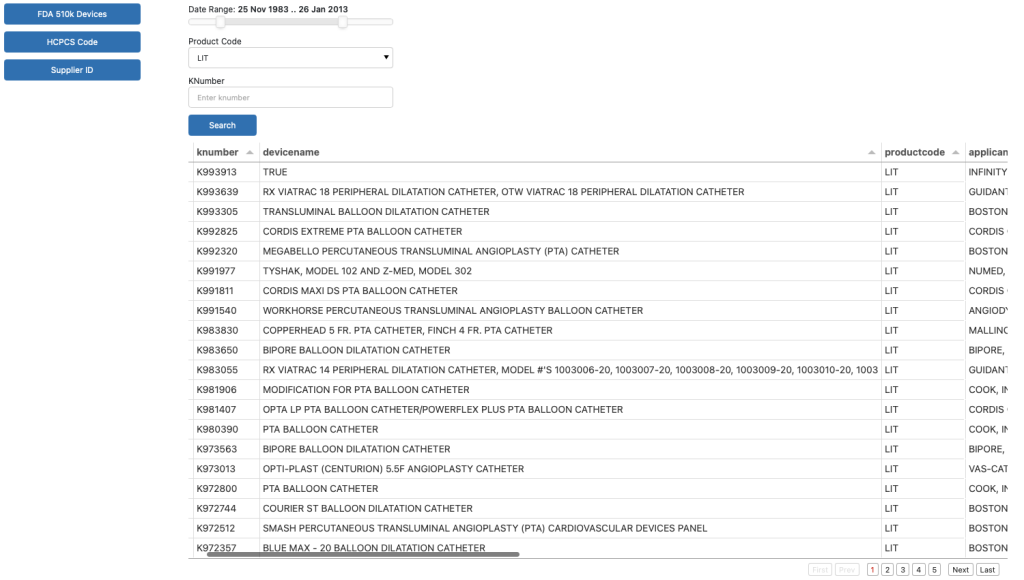
Below please find a screenshot of our internal tool for selecting types of data to integrate into customer’s systems.
Latest posts
-
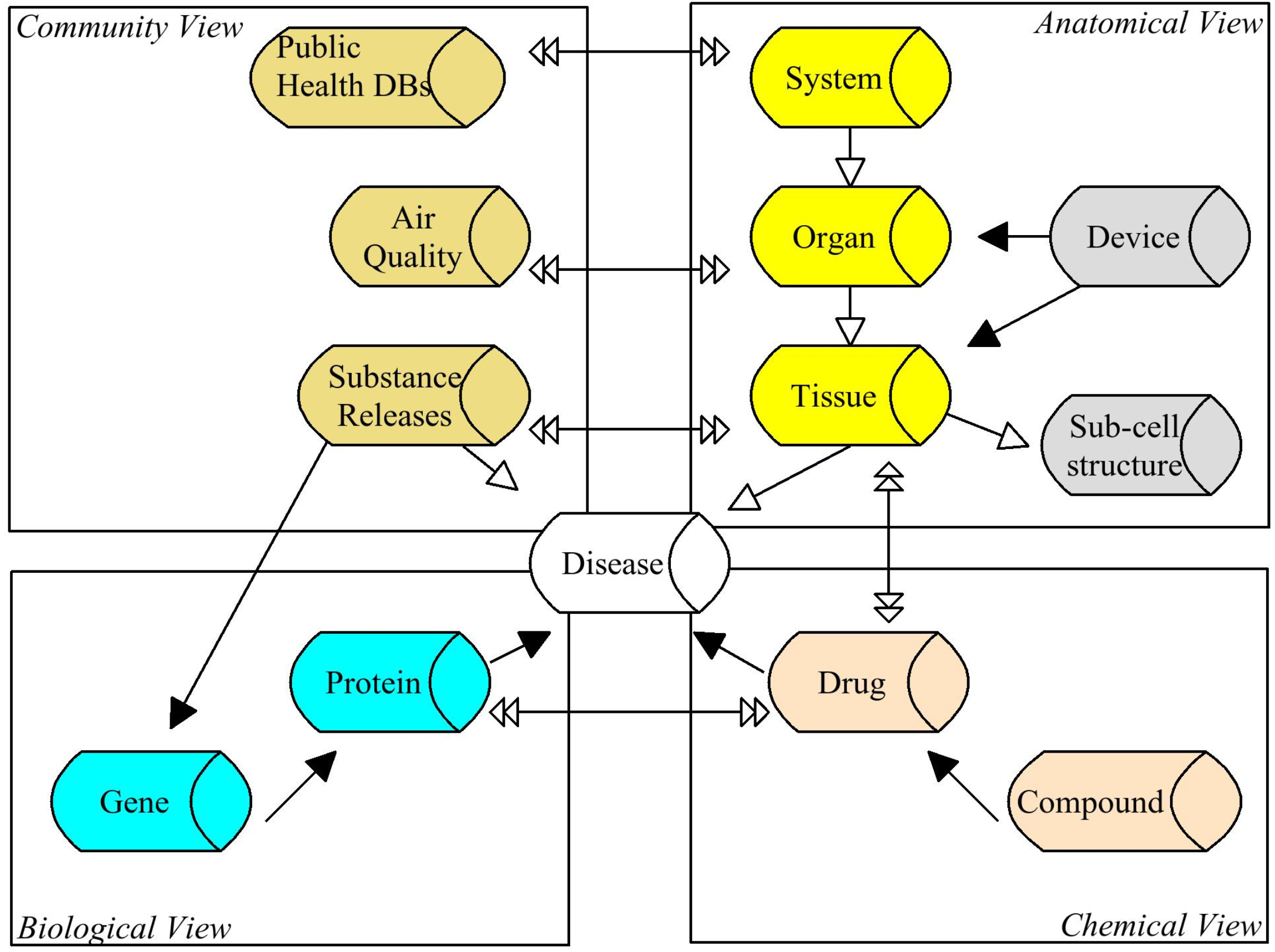
LifeSciences Design Platform
BlogSubscribe
-
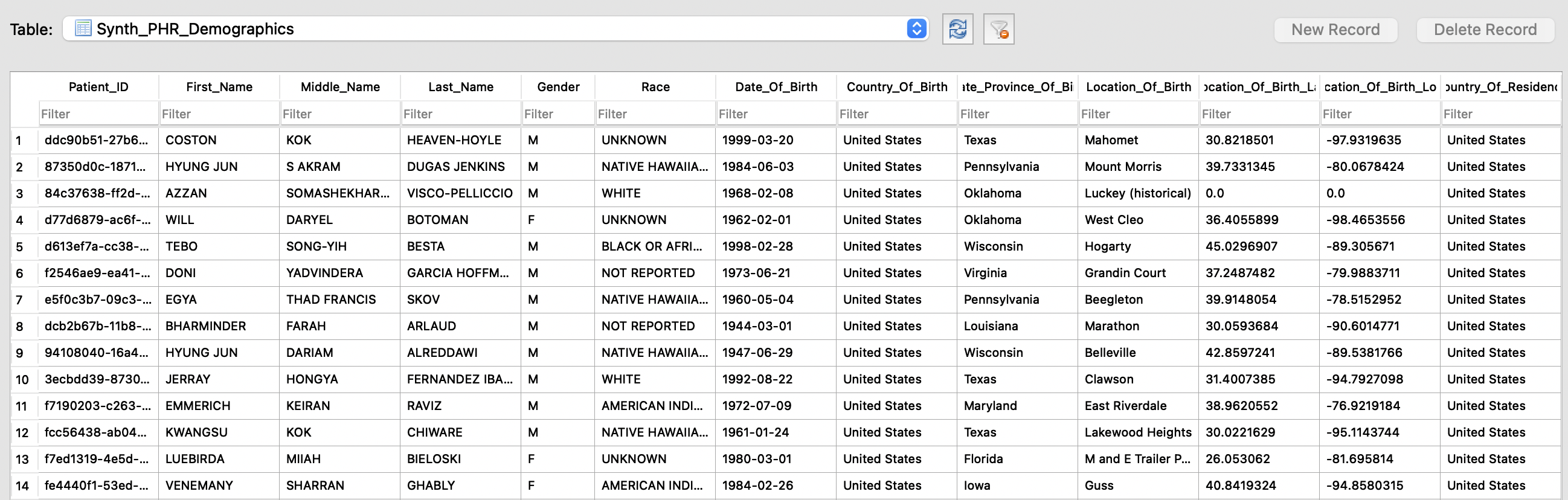
Customized Synthetic Data
In this article we cover how DataSDR generates customized Synthetic Data to improve your software development and debugging processes. You…
-
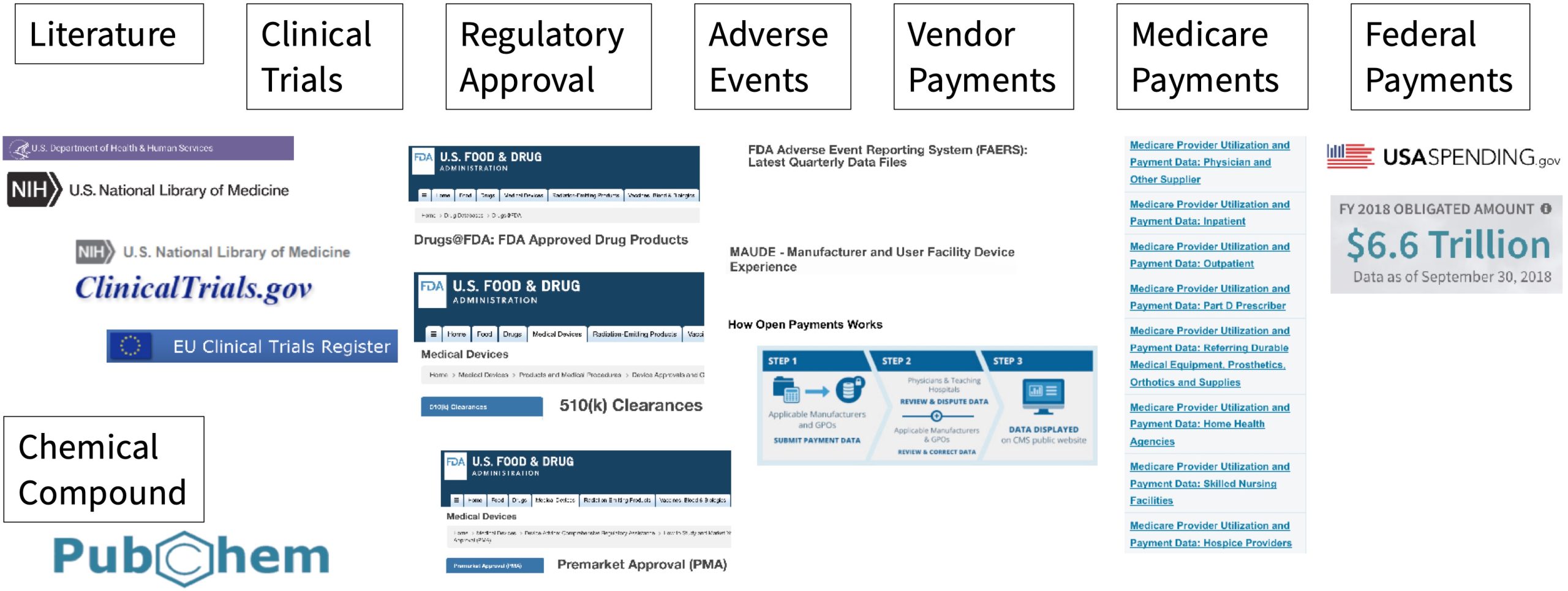
Using Open Data to Improve LLMs
In this article we’ll explore how Open Data can be used to improve Large Language Models (“LLMs”). We wrote a…
